Answer: A. 3 mol
 3 mol Al:
3 mol Al:
Step-by-step explanation:
Te given balanced chemical equation is :
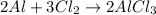
According to the stochiometry :
A: 3 mol
 3 mol Al:
3 mol Al:
3 moles of chlorine reacts with 2 moles of aluminium
B. 2 mol
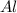 3 mol
3 mol
 :
:
2 moles of aluminium reacts with 3 moles of chlorine
C. 2 mol
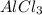 2 mol Al
2 mol Al
2 mol of aluminium produces 2 mol of
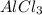
D. 3 mol
 2 mol
2 mol
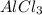
3 moles of chlorine produces with 2 moles of
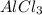
Thus the conversion factor not used for mole to mole calculations is 3 mol
 3 mol Al:
3 mol Al: