Answer:
ΔH vaporization of chloroform is 30.1kJ/mol
Step-by-step explanation:
It is possible to find ΔH of vaporization of certain compound knowing vapor pressure under 2 different absolute temperatures (In Kelvin) by using Clausius-Clapeyron equation:
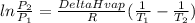
Where P is vapor pressure. R is gas constant (8.314J/molK) and T absolute temperature of 1, first state and 2, final state.
Absolute temperatures in the problem are:
T₁ = 24.1°C + 273.15 = 297.25K
T₂ = -6.3°C + 273.15 = 266.85K
Replacing:
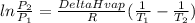
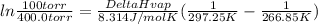
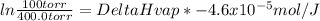
30073J/mol = 30.1kJ/mol = ΔHVap
ΔH vaporization of chloroform is 30.1kJ/mol