Answer:
d. [HI] > [H2]
Step-by-step explanation:
The explanation at equilibrium is shown below:-
Data provided
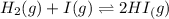
Initial concentration - -
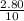 = 0.280 M
= 0.280 M
At equilibrium x x 0.280 - 2x
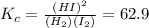
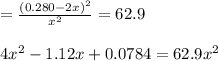
After solve the above equation we will get
x = 0.0282 M
Therefore at equilibrium
![[H_2] = [I_2] = x = 0.0282M\\\\](https://img.qammunity.org/2021/formulas/chemistry/high-school/cj7cmcfeia3y3hzx5psjba2o2u7bss2xei.png)
![[HI] = 0.280 - 2x = 0.2236 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/5orat3qqxoa0bebkdwenj4rdpwst687xe8.png)
Hence, the correct option is d.