Answer: The molality of this solution is 1.7 m
Step-by-step explanation:
Depression in freezing point:
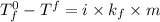
where,
 = freezing point of solution =
= freezing point of solution =
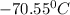
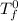 = freezing point of pure chloroform =
= freezing point of pure chloroform =
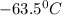
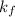 = freezing point constant of benzene =
= freezing point constant of benzene =
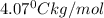
m = molality
i = Van't Hoff factor = 1 (for non-electrolyte)
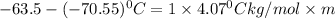
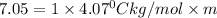
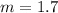
Thus the molality of this solution is 1.7 m