The given question is incomplete. The complete question is given here :
The equilibrium constant for the reaction is
 M.
M.
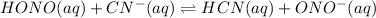
This value indicates that
A.
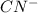 is a stronger base than
is a stronger base than

B. HCN is a stronger acid than HONO
C. The conjugate base of HONO is

D. The conjugate acid of CN- is HCN
Answer: A.
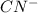 is a stronger base than
is a stronger base than

Step-by-step explanation:
Equilibrium constant is the ratio of product of the concentration of products to the product of concentration of reactants.
When
 ; the reaction is product favoured.
; the reaction is product favoured.
When
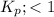 ; the reaction is reactant favored.
; the reaction is reactant favored.
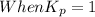 ; the reaction is in equilibrium.
; the reaction is in equilibrium.
As,
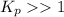 , the reaction will be product favoured and as it is a acid base reaction where
, the reaction will be product favoured and as it is a acid base reaction where
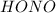 acts as acid by donating
acts as acid by donating
 ions and
ions and
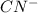 acts as base by accepting
acts as base by accepting

Thus
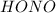 is a strong acid thus
is a strong acid thus
 will be a weak conjugate base and
will be a weak conjugate base and
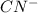 is a strong base which has weak
is a strong base which has weak
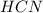 conjugate acid.
conjugate acid.
Thus the high value of K indicates that
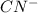 is a stronger base than
is a stronger base than
