Answer:
the mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water at 313 K is
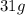
Step-by-step explanation:
To determine the mass of potassium nitrate needed to
produce a saturated solution of potassium nitrate in 50 grams of water at 313 K?, then it implies that we need to determine the
solubility of KNO3 is 62g KNO3 in 100g H2O at 313K. And this simply the amount of solute that when it dissolved in that water, then the water will not be able to take more solute again which means it has been saturated.that is the maximum quantity that the water can take at 313K.
If the solubility of KNO3 is 62g KNO3 in 100g H2O at temperature 313K
Then 50 g of water contains potassium nitrate = (62/100 X 50) at 313k = 31g
Therefore, the mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water at 313 K is
31g