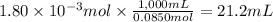Answer:
21.2 mL
Step-by-step explanation:
Step 1: Write the balanced equation.
NaOH + HBr ⇒ NaBr + H₂O
Step 2: Calculate the reacting moles of HBr
25.0 mL of 0.0720 M hydrobromic acid react.
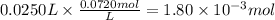
Step 3: Calculate the reacting moles of NaOH
The molar ratio of NaOH to HBr is 1:1. The reacting moles of NaOH are 1/1 × 1.80 × 10⁻³ mol = 1.80 × 10⁻³ mol.
Step 4: Calculate the required volume of NaOH