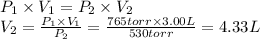Answer:
4.33 L
Step-by-step explanation:
Step 1: Given data
Initial volume of the balloon (V₁): 3.00 L
Initial pressure of the balloon (P₁): 765 torr
Final volume of the balloon (V₂): ?
Final pressure of the balloon (P₂): 530 torr
Step 2: Calculate the final volume of the balloon
If we consider Helium to behave as an ideal gas, we can calculate the final volume of the balloon using Boyle's law.