Answer:
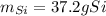
Step-by-step explanation:
Hello,
In this case, for the undergoing balanced chemical reaction:
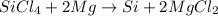
We must first identify the limiting reactant given the 225 g of SiCl4 and 101 g of Mg. Thus, we compute the available moles of SiCl4:
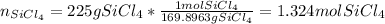
Next, by using the 1:2 mole ratio between SiCl4 and Mg, we compute the moles of SiCl4 consumed by 101 g of Mg:
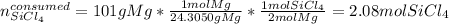
Thus, since less moles of SiCl4 are available, we can infer it is the limiting reactant whereas the Mg is in excess. In such a way, the produced grams of Si are computed considering the 1:1 molar ratio between SiCl4 and Si:
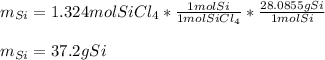
Best regards.