Answer:
Step-by-step explanation:
C₈H₁₆ + 12O₂ = 8 CO₂ + 8H₂O.
a )
Heat of formation of C₈H₁₆
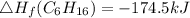
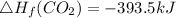
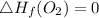
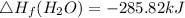
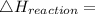 8 x - 393.5 - 8 x 285.82 + 174.5x 1
8 x - 393.5 - 8 x 285.82 + 174.5x 1
= - 5260.06 kJ
b ) Energy required = 2.905 x 10¹⁵kJ
moles of C₈H₁₆ require to be burnt
= 2.905 x 10¹⁵ / 5260.06
= 55.23 x 10¹⁰ moles
= 55.23 x 10¹⁰ x mol weight of C₈H₁₆ g
= 55.23 x 10¹⁰ x 112 g
= 6185.5 x 10¹⁰ g
= 6185.5 x 10⁷ kg
c )
No of litres of CO₂ produced at NTP = 8 x 22.4 x 55.23 x 10¹⁰ L
= 9897.22 x 10¹⁰ L
At 1520 mm of Hg pressure and 250°C
volume of CO₂
= 9897.22 x 10¹⁰ x 760 x ( 273 + 250) / ( 1520 x 273 )
= 9480.3 x 10¹⁰ L .