Answer:
The pressure of the gas sample will be 0.954 atm.
Step-by-step explanation:
Boyle's law states that the pressure of a gas in a closed container is inversely proportional to the volume of the container, when the temperature is constant. That is, if the pressure increases, the volume decreases; conversely if the pressure decreases, the volume increases.
Boyle's law is expressed mathematically as:
Pressure * Volume = constant
o P * V = k
To determine the change in pressure or volume during a transformation at constant temperature, the following is true:
P1 · V1 = P2 · V2
That is, the product between the initial pressure and the initial volume is equal to the product of the final pressure times the final volume.
In this case:
- P1= 0.609 atm
- V1= 19.9 L
- P2=?
- V2= 12.7 L
Replacing:
0.609 atm* 19.9 L= P2* 12.7 L
Solving:
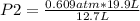
P2= 0.954 atm
The pressure of the gas sample will be 0.954 atm.