Answer:
0.5 M
Step-by-step explanation:
First, let us look at the balanced equation of the reaction.

The solute formed is
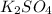 .
.
Recall that: mole = molarity x volume
Hence,
50 ml, 1.00 M H2SO4 = 0.05 x 1 = 0.05 mole
50 ml, 2.0 M KOH = 0.05 x 2 = 0.1 mole
From the equation
1 mole of H2SO4 reacts with 2 moles of KOH to give 1 mole of K2SO4.
Hence,
0.05 mole H2SO4 reacting with 0.1 mole KOH will give 0.05 mole
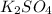 .
.
Also recall that: concentration = mole/volume
Total volume of resulting solution = 50 ml + 50 ml = 100 ml or 0.1 liter
Concentration of
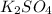 = mole of
= mole of
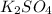 /volume of resulting solution
/volume of resulting solution
= 0.05/0.1 = 0.5 M
The concentration of the resulting solute = 0.5 M