Answer:
Sn1 mechanism reaction
Step-by-step explanation:
In this case, we have to start with the protonation of the "OH" group by the attack of the lone pairs in the alcohol group to the "H" in the HBr producing a positive charge in the oxygen. Then, water is produced and a carbocation is generated. This carbocation can be stabilized by a hydride shift. We can move a hydrogen atom to the positive charge and we will obtain a tertiary carbocation. Finally, the
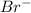 will attack to produce the final halide.
will attack to produce the final halide.
See figure 1.
I hope it helps!