Answer:
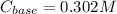
Step-by-step explanation:
Hello,
In this case, we can evidence that when calcium hydroxide solution reacts with hydrochloric acid solution, the balanced neutralization reaction turns out:
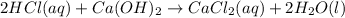
Moreover, the concentration of neutralized calcium hydroxide can be computed by using the 2:1 mole ratio between the base and the acid:
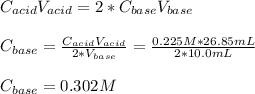
Regards.