Answer:
The first ionization energy is defined as
- Energy which is required to pull out one mole of the outermost shell's electrons in a neutral atom from one mole of gaseous atoms to produce 1 mole of gaseous ions each with a charge of 1+.
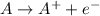 , where A is any neutral atom.
, where A is any neutral atom.- In the periodic table, Its value decreases from top to bottom in groups and increases from left to right across a particular period.
- Helium has the largest first ionization energy.
- Francium has one of the lowest.