Answer: The
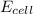 is 0.63 V
is 0.63 V
Step-by-step explanation:
In the given reaction :
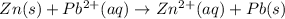
Here Zn undergoes oxidation by loss of electrons, thus act as anode. Lead undergoes reduction by gain of electrons and thus act as cathode.
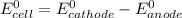
Where both
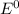 are standard reduction potentials.
are standard reduction potentials.
![E^0_([Pb^(2+)/Pb])= -0.13V](https://img.qammunity.org/2021/formulas/chemistry/high-school/unz0ouavml3f17wozshuzxsr9cqyl1eflb.png)
![E^0_([Zn^(2+)/Zn])=-0.76V](https://img.qammunity.org/2021/formulas/chemistry/high-school/olc4lf0brskzrvcslfp2n6xddpo3pietrg.png)
![E^0_(cell)=E^0_([Pb^(2+)/Pb])- E^0_([Zn^(2+)/Zn])](https://img.qammunity.org/2021/formulas/chemistry/high-school/bkwlhu70w76jmuwaleg599k30xfo9t02zt.png)
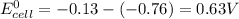
Thus the
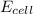 is 0.63 V
is 0.63 V