Answer:
3.1 L
Step-by-step explanation:
Step 1: Given data
- Mass of oxygen (m): 3.1 g
Step 2: Calculate the moles of oxygen
The molar mass of oxygen is 32.00 g/mol.
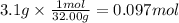
Step 3: Calculate the volume of the container
We will use the ideal gas equation.
P × V = n × R × T
V = n × R × T / P
V = 0.097 mol × (0.0821 atm.L/mol.K) × 390 K / 1.00 atm
V = 3.1 L