Answer:
Approximately 100 °C.
Step-by-step explanation:
Hello,
In this case, since the entropy of vaporization is computed in terms of the heat of vaporization and the temperature as:
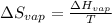
We can solve for the temperature as follows:
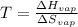
Thus, with the proper units, we obtain:
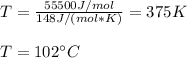
Hence, answer is approximately 100 °C.
Best regards.