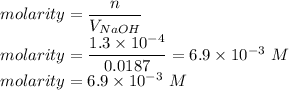Answer:
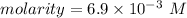
Step-by-step explanation:
We know that , the reaction of HCl and NaOH is given as follows
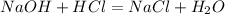
Given that
Pressure = 111 mm Hg
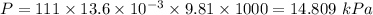
Temperature = 20°C
T=20+273=293 K
Volume= 221 m L
V=0.221 L
Number of moles of HCl is given as follows
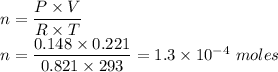
From the above reaction we can say that
Number of moles of HCl=Number of moles of NaOH
Volume of NaoH is given as follows
V=18.7 = 0.0187 L
Therefore molarity