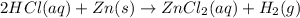Answer:
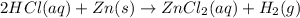
Step-by-step explanation:
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products formed must be equal to the mass of reactants taken.
In order to get the same mass on both sides, the atoms of each element must be balanced on both sides of the chemical equation.
A single replacement reaction is one in which a more reactive element displaces a less reactive element from its salt solution.
The salts which are soluble in water are designated by symbol (aq) and those which are insoluble in water and remain in solid form are represented by (s) after their chemical formulas. Gases are represented by (g) after their chemical formulas.
Thus 2 hydrogen chloride molecules in aqueous solution react with solid zinc. The reaction produces zinc chloride in aqueous solution and hydrogen gas is represented as :