Answer: 0.938 moles of
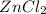 will be produced.
will be produced.
Step-by-step explanation:
To calculate the moles, we use the equation:
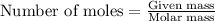
moles of zinc:
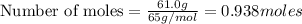
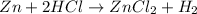
As HCl is in excess , zinc is the limiting reagent and it limits the formation of product.
According to stoichiometry :
1 mole of Zn produce = 1 mole of
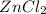
Thus moles of Zn produce =
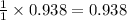 moles of
moles of
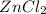
Thus 0.938 moles of
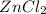 will be produced.
will be produced.