Answer:
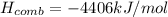
Step-by-step explanation:
Hello,
In this case, the enthalpy of combustion is understood as the energy released when one mole of fuel, in this case octene, is burned in the presence of oxygen and is computed with the enthalpies of formation of the fuel, carbon dioxide and water as shown below (oxygen is circumvented as it is a pure element):
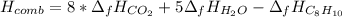
Thus, since we already know the enthalpy of combustion of the fuel, for carbon and water we have -393.5 and -241.8 kJ/mol respectively, thereby, the enthalpy of combustion turns out:
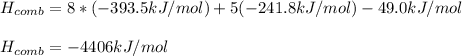
Best regards.