Answer:
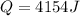
Step-by-step explanation:
Hello,
In this case, the involved heat in this heating process is considered to be computed via:
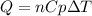
Whereas we assume a constant molar specific heat of helium which is 20.77 J/(mol*K), thus, the transferred energy in the form of heat turns out:
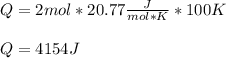
Regards.