Answer:
Intermediate.
Step-by-step explanation:
Hello,
In this case, we can rewrite the steps as:
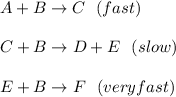
Thus, we can notice that in the fast step, C is present as a product but after that is consumed in the slow step, for that reason, and by cause of its formation-consumption behavior, it is properly described as an intermediate as it is not neither a starting-up substance (reactant in the first step) nor a final substance (product in the final step).
Best regards.