Answer:
The final temperature = 1007.26 K
The work done = 12016
Step-by-step explanation:
For isentropic compression, we have;
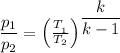
Where
p₁ = 1 bar
p₂ = 30 bar
T₁ = 160°C =
T₂ = The final temperature
K for steam = 1.33
T₂ = 433.15/(1/30)^(0.33/1.33) = 1007.26 K
The work done = m×
 ×(T₂ - T₁)
×(T₂ - T₁)
The work done per kilogram of steam =
 ×(T₂ - T₁)
×(T₂ - T₁)
Taking the average
 value, we have;
value, we have;
 at (1007.26 + 433.15)/2 = 2.080 + (2.113-2.080)×20.205/50 = 2.093 kJ/(kg·K)
at (1007.26 + 433.15)/2 = 2.080 + (2.113-2.080)×20.205/50 = 2.093 kJ/(kg·K)
Which gives = 2.093*(1007.26 -433.15) = 1201.6 kJ