Answer:
Catalyst
Step-by-step explanation:
For the reaction:
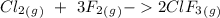
We have a main observation: When platinum is added the reaction goes faster. With this in mind, we have to remember the kinetic equilibrium theory. In figure 1, we have an energy diagram. In which we have an specific energy for the reagents and the products. When the reaction takes place, the reaction has to must go through an energy peak. This energy peak is called "activation energy". When platinum is added the activation energy decreases and the reaction can go faster. Therefore, platinum is a "catalyst", a substance with the ability to reduce the activation energy.
I hope it helps!