Answer:
The reaction quotient (Q) before the reaction is 0.32
Step-by-step explanation:
Being the reaction:
aA + bB ⇔ cC + dD
![Q=([C]^(c) *[D]^(d) )/([A]^(a)*[B]^(b) )](https://img.qammunity.org/2021/formulas/chemistry/college/oevgajish7f4ly6baf3bs5jrbscjh9c1t9.png)
where Q is the so-called reaction quotient and the concentrations expressed in it are not those of the equilibrium but those of the different reagents and products at a certain instant of the reaction.
The concentration will be calculated by:
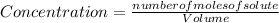
You know the reaction:
PCl₅ (g) ⇌ PCl₃(g) + Cl₂(g).
So:
![Q=([PCl_(3) ] *[Cl_(2) ] )/([PCl_(5) ])](https://img.qammunity.org/2021/formulas/chemistry/college/d9un0rpv4zmph7htoosfzxvfrl9m6wpsp8.png)
The concentrations are:
- [PCl₃]=
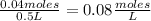
- [Cl₂]=
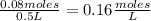
- [PCl₅]=
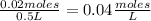
Replacing:
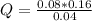
Solving:
Q= 0.32
The reaction quotient (Q) before the reaction is 0.32