Answer:
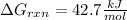
Step-by-step explanation:
In this case, for the dissociation of hypochlorous acid, we know that the acid dissociation constant (Ka) is 2.9x10⁻⁸, which is related with the Gibbs free energy as shown below:
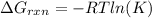
But in this case K is just Ka, therefore, at 296 K, it turns out:
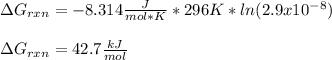
Such result, means that the reaction is nonspontaneous at the given temperature, it means it is not favorable (not easily occurring).
Best regards.