Answer:
Step-by-step explanation:
Let the monoprotic acid be HX
HX ⇄ H⁺ + X⁻
pH = 2.53
Hydrogen ion concentration
![[ H^+]=10^(-2.53)](https://img.qammunity.org/2021/formulas/chemistry/college/97vgx8tjrz0a9ip6w6agcs03n8fta8oz1g.png)
![[ X^-]=10^(-2.53)](https://img.qammunity.org/2021/formulas/chemistry/college/bnww0mbs0ft65nc8n4lrqcdwb6k9cnvtdq.png)
Concentration of undissociated acid will remain almost the same as it is a weak acid
So
Ka = concentration of H⁺ x concentration of Cl⁻ / concentration of acid
= [ H⁺] x [Cl⁻ ] / [ HX]
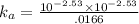
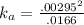
= 5.24 x 10⁻⁴ M .