Answer:
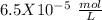
Step-by-step explanation:
For this question, we have to start with the ionization equation for
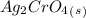 , so:
, so:
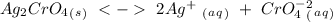
With this in mind we can write the Ksp expression:
![Kps~=~[Ag^+]^2[CrO_4^-^2]](https://img.qammunity.org/2021/formulas/chemistry/college/hhub69nf03juovai5dmjulvww19ywdeoki.png)
Additionally, for every mole of
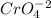 formed, 2 moles of
formed, 2 moles of
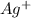 are formed. We can use "X" for the unknown concentration of each ion, so:
are formed. We can use "X" for the unknown concentration of each ion, so:
![[CrO_4^-^2]~=~X](https://img.qammunity.org/2021/formulas/chemistry/college/9kr1j23g13c6d9gfyhasfzf6anuytdz9ge.png) and
and
![[Ag^+]~=~2X](https://img.qammunity.org/2021/formulas/chemistry/college/8fiaxu7ubevzybdsikfra6uaau2s7wr3ng.png)
Now, we can plug the values into the Ksp expression:
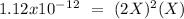
Now we can solve for "X" :
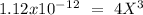
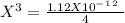
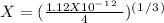
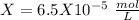
I hope it helps!