Answer:
The Explanation is described below:-
Step-by-step explanation:
The purpose of the emission of an element with an atomic number higher than 83 mostly during the disintegration of the nucleus is that if the alpha particle is released, the recently established nuclei or the daughter would be closer to the stability band.
In other terms, in the case of an alpha particle explosion, the daughter nuclei would be more stable than the parent nuclei.
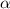 particles: Such particles are called alpha. They are basically divalent helium cations or helium nuclei with two protons and two neutrons. It is portrayed by
particles: Such particles are called alpha. They are basically divalent helium cations or helium nuclei with two protons and two neutrons. It is portrayed by
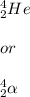
The Stability band is the area in the graph that is plotted between the number of protons and the number of protons representing the stable nucleus.