Answer:
121.67 g is to be added to 500 g of water
Step-by-step explanation:
Given that:
Pressure = 750 mmHg
Temperature T₁= 99.63⁰C = (273 + 99.63 ) = 372.63K
mass of water = 500 g
Temperature T₂ = 100⁰C = ( 273 + 100) K = 373 K
where;
Kb for water 0.52 K Kg mol-1
For sucrose; C₁₂ H₂₂ O₁₁
Molar mass = ( 12 × 12 )+ ( 1 × 22 ) + ( 16 × 11 )
Molar mass = 342 g/mol
ΔT = T₂ - T₁
ΔT = (373 - 372.63)K
ΔT = 0.37 K
∴ the amount of sucrose to be added to 500 g of water is:
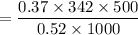
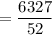
= 121.67 g
Thus; 121.67 g is to be added to 500 g of water