Answer:
The concentration of OH⁻ in the mixture is 0.05 M
Step-by-step explanation:
The reaction of neutralization between HCl and NaOH is the following:
H⁺(aq) + OH⁻(aq) ⇄ H₂O(l)
The number of moles of HCl is:
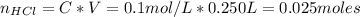
Similarly, the number of moles of NaOH is:
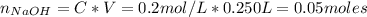
Now, from the reaction of HCl and NaOH we have the following number of moles of NaOH remaining:
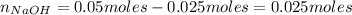
Finally, the concentration of OH⁻ in the mixture is:
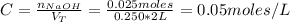
Therefore, the concentration of OH⁻ in the mixture is 0.05 M.
I hope it helps you!