Answer:
The molarity of the sulfuric acid in the solution is 1.77 M.
Step-by-step explanation:
The molarity of the sulfuric acid in the solution can be found using the following equation:
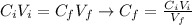
Where:
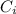 : is the initial concentration of the acid
: is the initial concentration of the acid
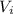 : is the initial volume of the solution = 22.6 cm³
: is the initial volume of the solution = 22.6 cm³
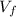 : is the final volume of the solution = 88.5 cm³
: is the final volume of the solution = 88.5 cm³
The initial concentration of the H₂SO₄ is:
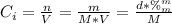
Where:
n: is the number of moles
m: is the mass
M: is the molar mass = 98.079 g/mol
d: is the density of the acid = 1.39 g/cm³
%: is the percent by mass = 49.0 %
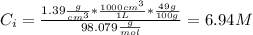
Finally, the final concentration of H₂SO₄ after the dilution is:
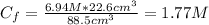
Therefore, the molarity of the sulfuric acid in the solution is 1.77 M.
I hope it helps you!