Answer:
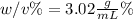
Step-by-step explanation:
Hello,
In this case, we first define the formula for the calculation of weight/volume percentage considering cobalt (II) fluoride as the solute, water the solvent and the both of them as the solution:
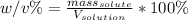
In such a way, since the mass of the solute is given as 6.04 g and the final volume of the solution 200 mL, the weight/volume percentage turns out:
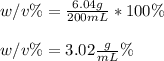
Regards.