Answer:

Step-by-step explanation:
Hello,
In this case, since the average rate of reaction is related with the consumption of A which has an stoichiometric coefficient of 1, the rate of formation of B will be:
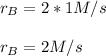
By cause of the stoichiometric coefficient of B which doubles the average rate.
Best regards.