Answer:
The volume of the balloon after 38.0 hours is 99.55 L
Step-by-step explanation:
Given;
volume of helium in the weather balloon, V = 110 L
initial mole of the gas, n = 4 mol
the rate leak of the gas, dn/dt = 10 mmol/hr
After 38.0 hours, the moles of the gas lost = 38hr x 10 x 10⁻³ mol/hr = 0.38 mol
the moles of the gas lost = 0.38 mol
Number of moles of helium in balloon after 38.0 hours = 4 mol - 0.38 mol
= 3.62 mol
Volume of helium in balloon after 38.0 hours ;
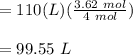
Therefore, the volume of the balloon after 38.0 hours is 99.55 L