Answer:
The liquids are TOLUENE because the equilibrum vapor above it will be flammable ( D )
Step-by-step explanation:
Liquids stored at : 1 atm , 25⁰c and they are vented with air
Determining whether the equilibrum vapor above the liquid will be flammable
We can determine this by using Antoine equation to calculate saturation vapor pressure also apply Dalton's law to determine the volume % concentration of air and finally we compare answer to flammable limits to determine which liquid will be flammable
A) For acetone
using the Antoine equation to calculate saturation vapor pressure
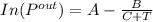
values gotten appendix E ( chemical process safety (3rd edition) )
A = 16.6513
B = 2940.46
C = -35.93
T = 298 k input values into Antoine equation
therefore ;
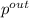 = 228.4 mg
= 228.4 mg
calculate volume percentage using Dalton's law
= V% = (saturation vapor pressure / pressure ) *100
= (228.4 mmHg / 760 mmHg) * 100 = 30.1%
The liquid is not flammable because its UFL = 12.8%
B) For Benzene
using the Antoine equation to calculate saturation vapor pressure
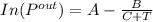
values gotten appendix E ( chemical process safety (3rd edition) )
A= 15.9008
B = 2788.52
C = -52.36
T = 298 k input values into the above equation
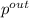 = 94.5 mmHg
= 94.5 mmHg
calculate volume percentage using Dalton's law
V% = (saturation vapor pressure / pressure ) *100
= (94.5 / 760 ) * 100 = 12.4%
Benzene is not flammable under the given conditions because its UFL =7.1%
C) For cyclohexane
using the Antoine equation to calculate saturation vapor pressure
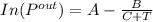
values gotten appendix E ( chemical process safety (3rd edition) )
A = 15.7527
B = 2766.63
c = -50.50
T = 298 k
solving the above equation using the given values
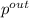 = 96.9 mmHg
= 96.9 mmHg
calculate volume percentage using Dalton's law
V% = (saturation vapor pressure / pressure ) *100
= ( 96.9 mmHg /760 mmHg) * 100 = 12.7%
cyclohexane not flammable under the given conditions because its UFL= 8%
D) For Toluene
using the Antoine equation to calculate saturation vapor pressure
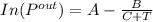
values gotten from appendix E ( chemical process safety (3rd edition) )
A = 16.0137
B = 3096.52
C = -53.67
T = 298 k
solving the above equation using the given values
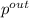 = 28.2 mmHg
= 28.2 mmHg
calculate volume percentage using Dalton's law
V% = (saturation vapor pressure / pressure ) *100
= (28.2 mmHg / 760 mmHg) * 100 = 3.7%
Toluene is flammable under the given conditions because its UFL= 7.1%