Answer:
( About ) 0.03232 M
Step-by-step explanation:
Based on the units for this reaction it should be a second order reaction, and hence you would apply the integrated rate law equation "1 / [X] = kt + 1 / [
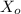 ]"
]"
This formula would be true for the following information -
{
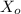 = the initial concentration of X, k = rate constant, [ X ] = the concentration after a certain time ( which is what you need to determine ), and t = time in minutes }
= the initial concentration of X, k = rate constant, [ X ] = the concentration after a certain time ( which is what you need to determine ), and t = time in minutes }
________
Therefore, all we have left to do is plug in the known values. The initial concentration of X is 0.467 at a time of 0 minutes, as you can tell from the given data. This is not relevant to the time needed in the formula, as we need to calculate the concentration of X after 18 minutes ( time = 18 minutes ). And of course k, the rate constant = 1.6
1 / [X] = ( 1.6 )( 18 minutes ) + 1 / ( 0.467 ) - Now let's solve for X
1 / [X] = 28.8 + 1 / ( 0.467 ),
1 / [X] = 28.8 + 2.1413...,
1 / [X] = 31,
[X] = 1 / 31 = ( About ) 0.03232 M
Now for this last bit here you probably are wondering why 1 / 31 is not 0.03232, rather 0.032258... Well, I did approximate one of the numbers along the way ( 2.1413... ) and took the precise value into account on my own and solved a bit more accurately. So that is your solution! The concentration of X after 18 minutes is about 0.03232 M