Answer:
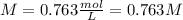
Step-by-step explanation:
Hello,
In this case, as the osmotic pressure (π) is widely known as a colligative property, we can see that the solution in this case is formed by water and tree sap, that is mathematically defined by:
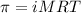
Thus, since tree sap is a covalent substance that is nonionizing, we can infer its van't Hoff factor to be 1, therefore, for the given osmotic pressure and temperature, we can compute the molar concentration (in molar units mol/L) as follows:
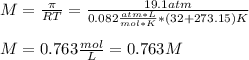
Best regards.