Answer:
52 g was lost as carbon dioxide to the atmosphere
Step-by-step explanation:
When lead carbonate is heated, it decomposes into two components:
1. Lead oxide
2. carbon dioxide
While the lead oxide remains a yellow solid in the heating container, the carbon dioxide escapes into the atmosphere as a gas. The equation of the reaction is as below:
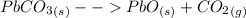
Hence, if a 150 g lead oxide is heated in a container and the final mass is 98 g, it means 52 g (150 - 98) of the total mass has been lost as carbon dioxide to the atmosphere.