Answer:
dimethoxy(phenyl)methanol
Step-by-step explanation:
For this question, we have to remember the mechanism of the Grignard reaction. In this case, phenylmagnesium bromide is our nucleophile, a carbo-anion is produced (step 1). Then this carbo-anion can attack the carbonyl group in the dimethyl carbonate, the double bond is delocalized into the oxygen producing a negative charge (step 2). Finally, with the addition of the hydronium ion (
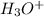 ), the anion can be protonated to produce the alcohol (dimethoxy(phenyl)methanol) (step 3).
), the anion can be protonated to produce the alcohol (dimethoxy(phenyl)methanol) (step 3).
See figure 1
I hope it helps!