Answer:
B) It must be a weak acid.
Step-by-step explanation:
If HA is a strong acid
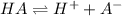
Now the pH value can be written as follows
![pH=-log[H^+]\\pH=-log(5* 10^(-2))\\pH=2* log5\\pH=1.4](https://img.qammunity.org/2021/formulas/chemistry/college/5k3jffy2c9ugrg9znqenns4dnp769aclty.png)
But given that acid HA has 2.3 pH value.
Therefore we can say that HA is weak acid.
Thus the answer will be option (B).
B) It must be a weak acid.