Answer:
2415.9g (corrected to 1 d.p.)
Step-by-step explanation:
(Take the atomic mass of Ga=69.7 and S=32.1)
Assuming Ga is the limiting reagent (because the question did not mention the amount of sulphur burnt),
From the balanced equation, the mole ratio of Ga:Ga2S3 = 4: 2 = 2: 1, which means, every 2 moles of Ga burnt, 1 mole of Ga2S3 is produced.
Using this ratio, let y be the no. of moles of Ga2S3 produced,
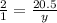
y = 20.5 / 2
= 10.25 mol
Since mass = no. of moles x molar mass,
the mass of Ga2S3 produced = 10.25 x (69.7x2 + 32.1x3)
= 2415.9g (corrected to 1 d.p.)