Answer:
15.9 g
Step-by-step explanation:
(Take the atomic mass of C=12.0, H=1.0, O=16.0)
no. of moles = mass / molar mass
no. of moles of octane used = 11.2 / (12.0x8 + 1x18)
= 0.0982456 mol
Since oxygen is in excess and octane is the limiting reagent, the no. of moles of H2O depends on the no. of moles of octane used.
From the balanced equation, the mole ratio of octane : water = 2:18 = 1: 9,
so this means, one mole of octane produced 9 moles of water.
Using this ratio, we can deduce that (y is the no. of moles of water produced):
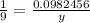
y = 0.0982456x9
y= 0.88421 mol
Since mass = no. of moles x molar mass,
mass of water produced = 0.88421 x (1.0x2+16.0)
=15.9 g