Answer:
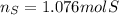
Step-by-step explanation:
Hello,
In this case, given the undergoing chemical reaction, we can see a 4:3 mole ratio between the consumed moles of gallium and sulfur respectively, therefore, the consumed moles of sulfur, from the 100.0 g of gallium (use its atomic mass) turn out:
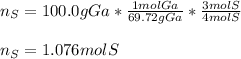
Best regards.