Answer: [H2SO4] = 0.5M;
[KOH] = 1M
Step-by-step explanation: Molarity is the solution concentration defined by:
molarity =
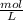 or M
or M
To determine the concentration of the mixture, find how many mols of each compound there are in the mixture:
50 mL = 0.05L
H2SO4
1 mol/L * 0.05L = 0.05mol
KOH
2mol/L * 0.05L = 0.1 mol
The mixture has a total volume of:
V = 50 + 50 = 100 mL = 0.1 L
The concentration of the resullting solutes:
[H2SO4] =
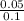 = 0.5 M
= 0.5 M
[KOH] =
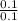 = 1 M
= 1 M
Concentration of H2SO4 is 0.5M while for KOH is 1M.