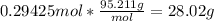Answer: 28.02 g
Step-by-step explanation:
The M stands for molarity. It is moles of solute/liters of solution. We can use the molarity to convert liters to mL, then make a proportion to find the grams.
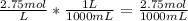
Now that we have molarity in moles and mL, we can use the 107mL to get moles.
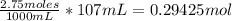
We would multiply moles by molar mass to get grams. The molar mass of magnesium chloride is 95.211 g/mol.