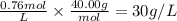Answer:
0.76 M
30 g/L
Step-by-step explanation:
Step 1: Given data
- Molarity of the acid (Ma): 0.175 M
- Volume of the acid (Va): 375 mL
- Molarity of the base (Mb): ?
- Volume of the base (Vb): 86 mL
Step 2: Calculate the molarity of the base
We will use the following expression.
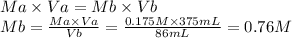
Step 3: Calculate the concentration of the base in g/L
The molar mass of NaOH is 40.00 g/mol.