Answer:
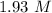
Step-by-step explanation:
We can start with the data given by the problem:
Mass of HF = 12.0 g
Volume of solution =
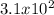 mL
mL
If we want to calculate the "concentration", we have to remember the molarity equation:
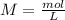
So, we need to know the moles and the litters if we want to calculate the molarity.
Calculation of Litters
We already have a volume value, but the value given by the problem has "mL". So, we have to do a conversion. In 1 L we have 1000 mL (1 L = 1000 mL), therefore:
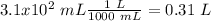
Calculation of moles
In this case, the compound is HF. So, we need to know the molar mass of HF if we want to do the conversion from grams to moles. The atomic mass of "F" is 18.99 g/mol and the atomic mass of "H" is 1 g/mol. So:
18.99 + 1 = 20 g /mol
In other words, 1 mol of HF is equal to 20 g (1 mol = 20 g HF). With this in mind, we can calculate the moles in the 12 g:
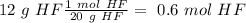
Calculation of the concentration of the solution
With these values know we can calculate the molarity:
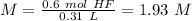
I hope it helps!